Introduction: Equitable representation of diverse racial and ethnic groups in oncology clinical trials continues to be a major challenge. Numerous initiatives designed to enhance diversity in oncology clinical trials are ongoing, including efforts at the U.S. Food and Drug Administration (FDA) such as the Oncology Center of Excellence's Project Equity. Given the multiple recent FDA approvals of CAR T-cell therapies for relapsed or refractory hematologic malignancies, we evaluated the enrollment rates of racial and ethnic groups in the CAR T-cell registrational trials submitted to the FDA.
Methods: We conducted a pooled, patient-level analysis of all registrational trials of CAR T-cell therapies submitted to the FDA to support approval for hematologic malignancies between 2017 and 2021. Datasets from approved licensing applications were pooled, and demographics and efficacy outcomes were analyzed descriptively.
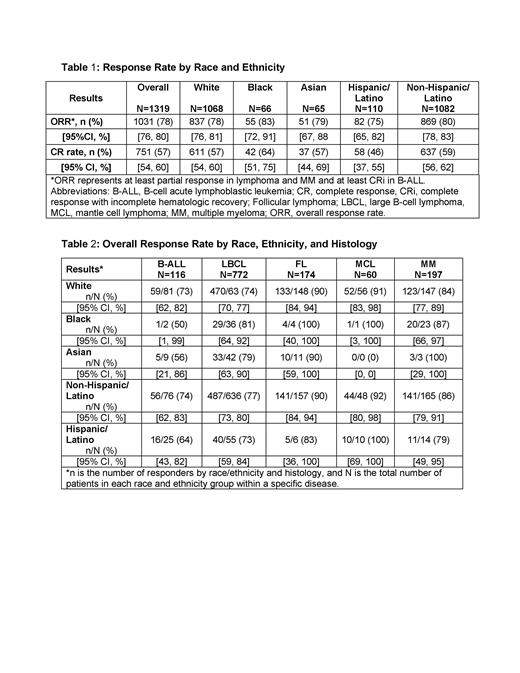
Results: A total of 13 registrational trials involving 6 autologous CAR T-cell products were analyzed, comprising 1,319 patients with hematologic malignancies. The median age at enrollment was 60 years (range: 3 to 86 years). Of the 1,319 patients, 81% (1068) were White, 5% (66) were Black, 5% (65) were Asian, 4% (48) were reported as Other, and 8% (110) were Hispanic or Latino. Race and ethnicity information was not reported for 5% (72) and 10% (127) of patients, respectively. The majority of the patients enrolled in the registrational trials were from the United States (80%), followed by Europe (11%) and Australia (2%). The hematologic malignancies included large B cell lymphoma (59%), multiple myeloma (15%), follicular lymphoma (13%), B cell acute lymphoblastic leukemia (9%), and mantle cell lymphoma (5%). Response rates by race, ethnicity, and histology are shown in Table 1 and Table 2.
Conclusions: Despite the majority of patients being enrolled in the U.S., racial/ethnic subgroups other than White and non-Hispanic/Latino were underrepresented in CAR T-cell registrational trials. This highlights the need to understand and overcome the continued barriers to CAR T-cell clinical trial enrollment across racial and ethnic groups. Although clinically meaningful efficacy was observed across these racial/ethnic groups and diseases, more data are needed to evaluate if efficacy outcomes vary across these subgroups. Work to describe safety and tolerability, particularly cytokine release syndrome (CRS) and neurotoxicity, in these racial and ethnic subgroups is ongoing. More effort is needed to better enhance diversity in CAR T-cell trials, especially given the expanding use of CAR T-cell therapies in the treatment of patients with hematologic malignancies.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal